Theory of scanning electron microscopy and X-ray microanalysis
From a historical point of view, the scanning electron microscope (SEM) and the electron probe micro analyzer (EPMA), also
known as electron microprobe (EMP), evolved separately. However, they are in fact very similar and differ mainly in the way
they are utilized. At the NHM the EPMA is used mainly for high precision WDS measurements and high resolution imagining at
high magnification, whereas the SEM is mainly used for imaging at moderate magnifications and quick EDS measurements.
In both instruments, the surface area to be examined, or the micro volume to be analyzed, is irradiated with a finely focused
electron beam. The electrons of the primary beam stimulate the sample to emit different types of electrons and photons that
can be collected by various detectors and used to image the sample surface as well as to perform chemical analysis.
Specimen – electron beam interaction
When a beam of accelerated electrons is focused on the surface of a specimen, strong elastic and inelastic scattering interactions
occur between high energetic free electrons and the atoms of the sample. As a result, several types of electrons and photons
are produced, the ones used in our labs are displayed in the following figure.
Secondary (SE) and Auger (AE) electrons are of low energy carrying information about topography and chemistry of the first
several tens to thousands atomic layers of the sample. Backscattered electrons (BSE) are elastic scattered high-energy particles,
providing information about the mean atomic number of the sampled volume. Additionally, these electrons can be used for electron
backscatter diffraction (EBSD) to evaluate the crystallographic orientation of different phases. Two different types of X-
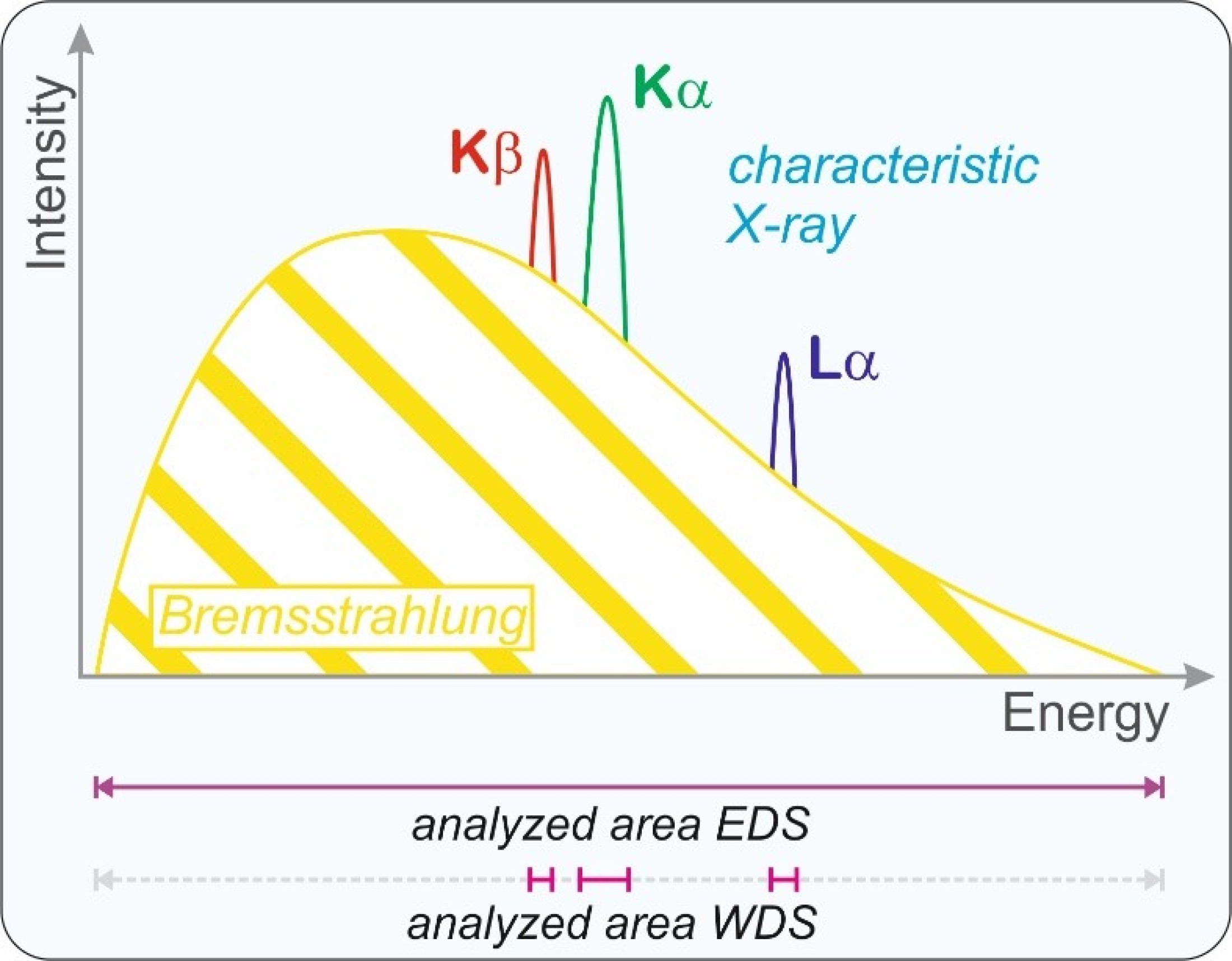
rays are produced in the sample, the low energy characteristic X-ray and the high energy Bremsstrahlung. The characteristic
X-ray provides information about the chemical composition of the analyzed volume. In addition to the different types of electrons
and X-rays light is emitted from the sample. The so-called Cathodoluminescence (CL) is used do get structural information
of the phases.
These electrons and photons are produced in specific excitation and escaping volumes in the sample. The size of the volume
is a function of the energy of incoming electrons (the higher the energy, the larger the volume) and the density of the sample
(the higher the density, the smaller the volume).
X-ray generation
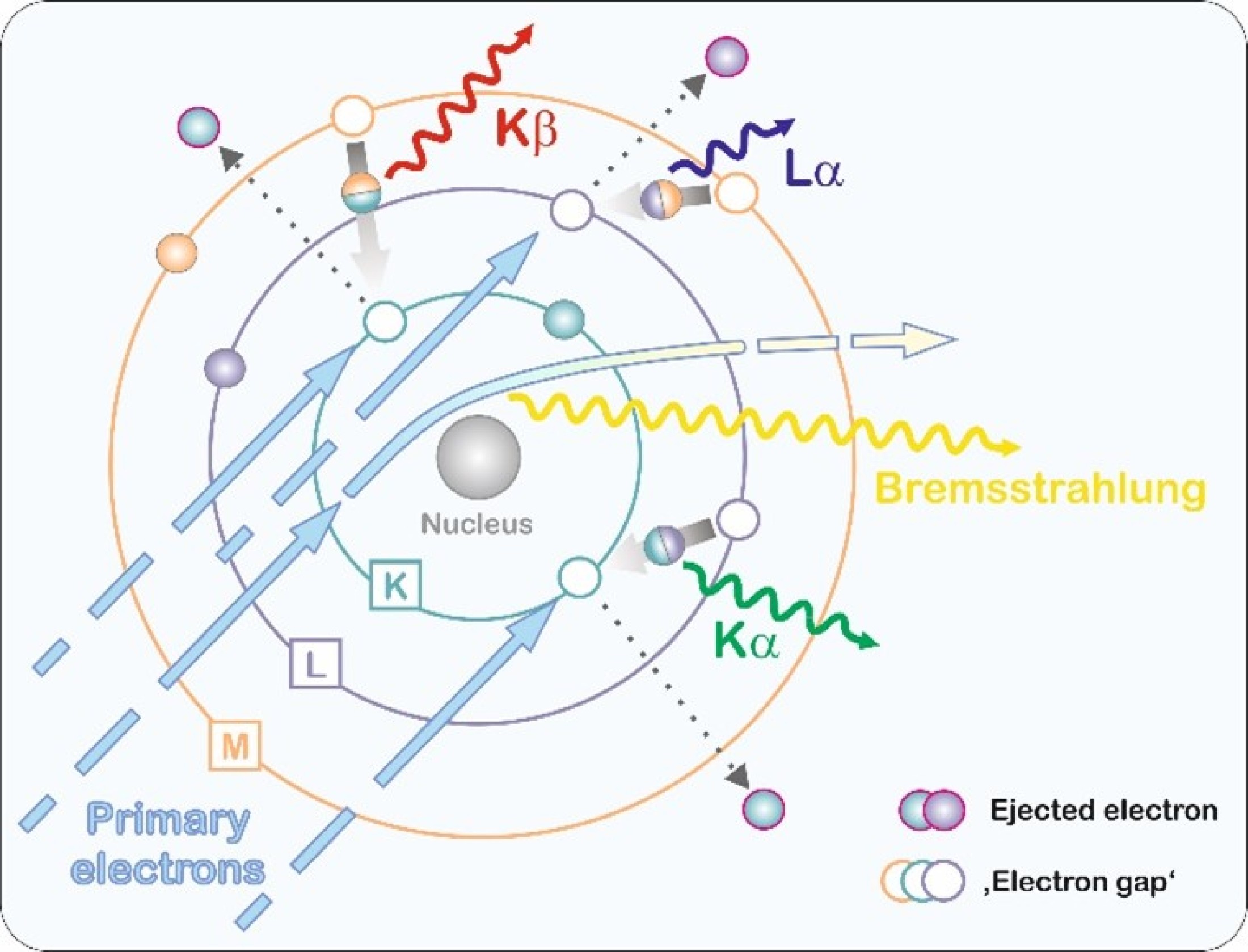
Characteristic X-ray generation in a sample is based on excitation of atoms. In EPMA and SEM systems, this is realized by
bombardment with electrons. An electron is knocked out of one of the inner shells of the atom. Such a state is unstable and
the resulting 'gap' is immediately filled by a higher-energy electron from an outer shell.